What We Do
Our laboratory was established in February 2005, with a mission to develop in human resources in both the industry and government who are equipped with up-to-date knowledge and ability to perform optimal drug evaluation. This laboratory is the first of its kind in Japan to provide educational opportunities and research platform for drug evaluation; ranging from natural science to social science, including medical needs, drug policy, and the legal system.
Overview
◆Educational Programs
We have been providing educational programs to undergraduate/ graduate students, researchers and also practitioners and professionals in government organizations, pharmaceutical companies, and medical institutions.
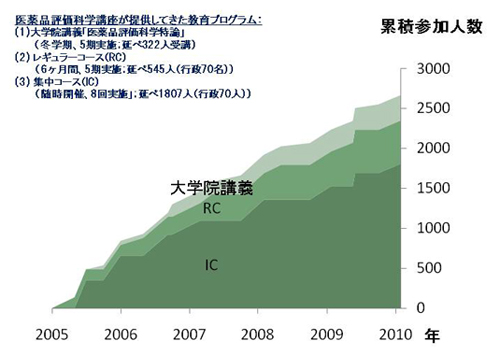
(1) Graduate School Lectures
The course of 15 lectures are designed to teach students and researchers adequate evaluation of drugs, and to recognize how pharmaceutical sciences works in developing new and efficient drugs that should benefit healthcare. Not only graduate school students but also many nondegree students from the industry or government to take this lecture
(2) Regular Course (RC)
This half-year course includes lectures and 2 sets of debates and group discussion. This course aims at an audience of governmental regulators, pre-clinical and clinical researchers in industry and institutions, and industrial managers in R&D. The objective of this course is to provide attendees with up-to-date knowledge on global guidelines, statistics and data management methodology, difficulties in efficacy/safety evaluation, and other topics that are of importance for efficient drug development and evaluation.
(3) Intensive Course (IC)
This 1 or 2 day educational course delivers a training program of international relevance on varying topics. The topics of concern are the bottlenecks of drug development, approached by various perspectives; medical, pharmaceutical, social, economical, and also industrial viewpoints.
◆Research activity
We have been successful in developing a broad cross-disciplinary
research field of both natural and social science. Our research utilizes various
methods to quantify and analyze the regulation and behaviors of various players
in drug development. The outputs of our research are not only presented in
academic gatherings and published as journal articles, but also used in
professional meetings, policy debates, and in discussions on drug policy within
political parties. The ultimate goal of our research is establishment of
regulatory science in drug evaluation of efficacy and safety.
(Please see "Research"for details)
Contact Us
TEL:03-5841-1692
TEL:03-5841-4788(RC)
FAX:03-5800-6949
PRS Secretary
