Establishing scientific drug evaluation
The goal of our research is to establish scientific principles and methods
in drug evaluation with societal perspectives in mind. Pharmaceutical research
and development (R&D), clinical development in particular, regulatory review
and approval of new drugs, and post marketing activities are our research
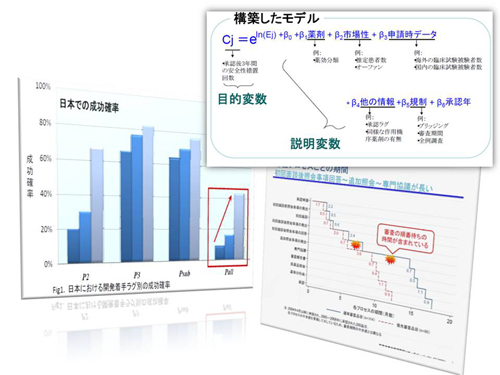
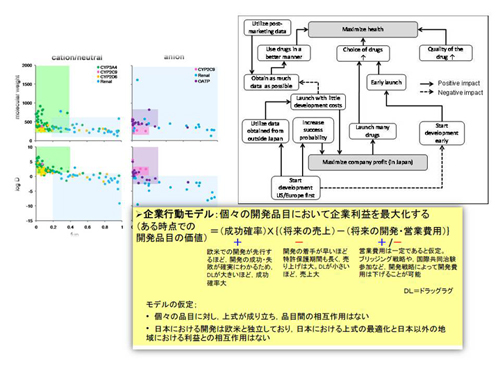
interests. We provide evidence on R&D efficiency, performance
and outcomes of regulations, and public health impact through rigorous analysis
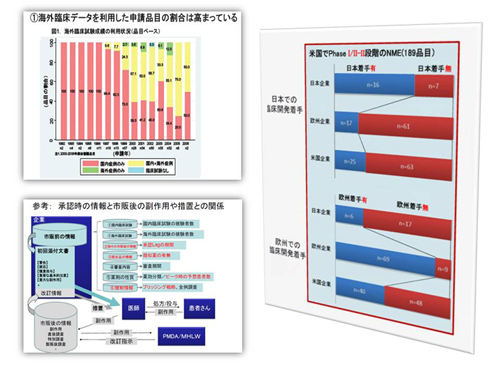
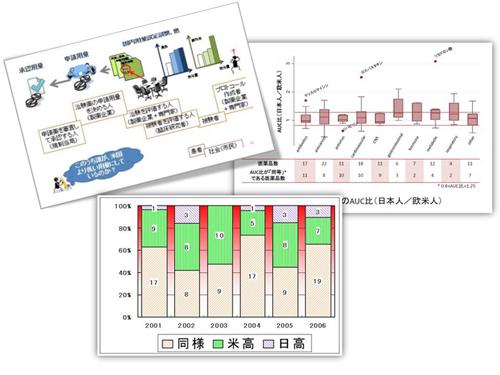
based on economic models. Conflicts in global pharmaceutical R&D, including
recent launch delay of new drugs in Japan and so-called ethnic differences,
are always high on our agenda.
Aside from the research activities, we also make efforts to develop human resources in both private and public sectors with up-to-date knowledge, ethics, and philosophy, and rationale in drug evaluation.
We offer lectures for graduate and undergraduate students, and a half-year training course for industry and regulatory professionals. We aim to secure transparency and social responsibility on drug regulation through our research and educational programs.
Our major research topics are as follows:
Aside from the research activities, we also make efforts to develop human resources in both private and public sectors with up-to-date knowledge, ethics, and philosophy, and rationale in drug evaluation.
We offer lectures for graduate and undergraduate students, and a half-year training course for industry and regulatory professionals. We aim to secure transparency and social responsibility on drug regulation through our research and educational programs.
Our major research topics are as follows:
(1) Economic and regulatory impact on global pharmaceutical R&D activities
(2) Regulatory review and approval of new drugs
(3) Roles of 'ethnic differences' in decisions of drug development
(4) Drug safety and regulatory environments




Contact Us
TELF03-5841-1692
TELF03-5841-4788iRCj
FAXF03-5800-6949
PRS Secretary
